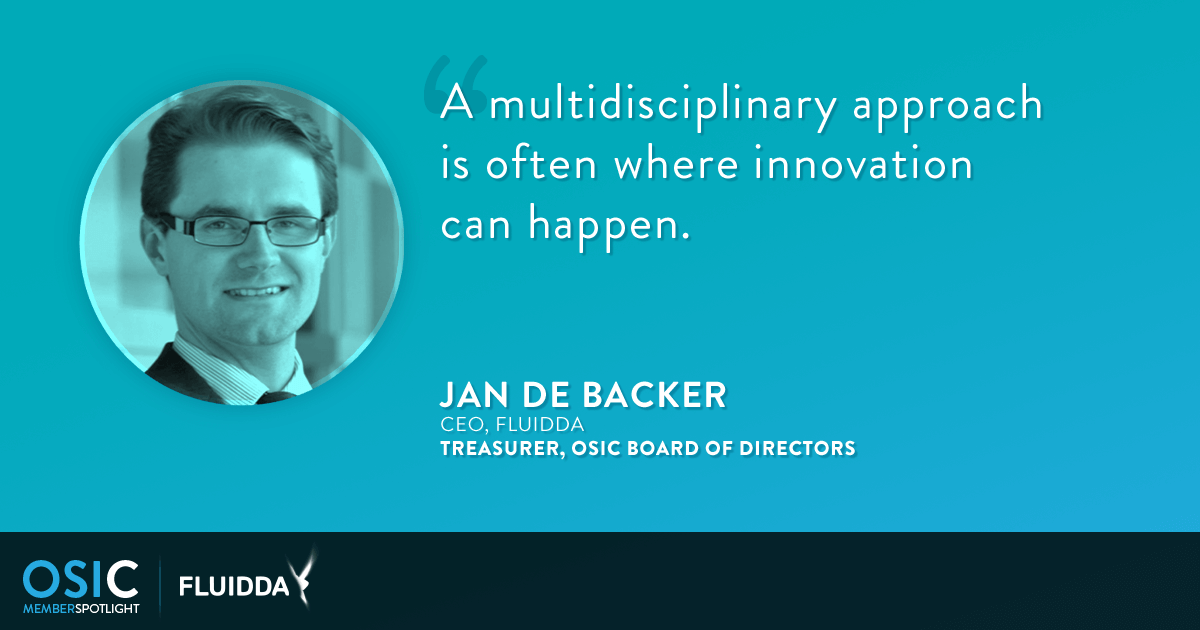
A Q&A with Jan De Backer
Founded in 2005, FLUIDDA provides respiratory solutions for better patient care. As a major player in the field of Quantitative Pulmonary CT, FLUIDDA leads the pack with its proprietary Functional Respiratory Imaging (FRI) technology. This technique combines HRCT scans and Computational Fluid Dynamics technology, which offers vast improvements by making clinical trials shorter, faster, and more cost effective.
FRI also helps patients and healthcare providers in offering a unique entry point in personalized medicine, by optimizing diagnosis, monitoring disease progression, and the effects of therapy. FLUIDDA’s goal is to improve the lives of people suffering from respiratory diseases by optimizing treatment pathways, reducing healthcare costs, and limiting the go-to-market time of respiratory drugs, pulmonology medical devices, and therapies. This passion for innovation is what drove FLUIDDA to become a founding member of OSIC. From the start, FLUIDDA has shared its expertise and learnings with us, and has been a key influence in helping shape our mission and drive our strategic direction. We spoke with FLUIDDA CEO Jan De Backer, who also serves as a member of our board of directors, about what first drew him to OSIC, the unique perspective that FLUIDDA brings to our organization, and where he sees OSIC five years down the line. Following is an excerpt from our conversation. What first drew you to OSIC? When I started FLUIDDA, my intention was to use medical imaging in combination with other insights and technologies (in our case, aerospace engineering, which is my background) to improve medical technology. This multidisciplinary approach is often where innovation can happen.
What unique perspective does FLUIDDA bring to the table?
We’ve been going through the motions for the last 15 years of bringing image-based technology closer to the patient and demonstrating where it is clinically valuable. We’ve been part of the FDA’s Critical Path Initiative (CPI) that approves biomarkers, so we know what the regulators want to see in terms of efficacy and clinical data. And we’ve built a business that grew organically for 13–14 years by 30% per year by working for pharmaceutical and medical device companies, indicating that there is a market fit for technology-driven companies in the respiratory space.
For people wondering if they should join OSIC, what would you say to them? What is the value in being a part of this organization?
OSIC is a tremendous resource, and not just because members have access to the Data Repository. What is equally as valuable is that members have access to other members. OSIC is a place where you can get guidance to help bring your technology closer to patients, and where you can learn how to be viable and sustainable.
If you had a crystal ball, what do you think OSIC will have accomplished in five years?
My hope is that in five years, one of the OSIC-created algorithms will have made it to clinical practice and will have significantly improved the fate of IPF patients. At the point where OSIC is now, that should be very doable. |
Get the latest OSIC news delivered right to your inboxNo spam. Just news.
By clicking the 'Subscribe to OSIC News' button, you consent to receive news and promotional materials from OSIC and agree to the processing of your personal data for this purpose as outlined in our Privacy Policy. You can withdraw your consent at any time.
|
Copyright © 2024 Open Source Imaging Consortium (OSIC). All rights reserved.

 RSS Feed
RSS Feed

